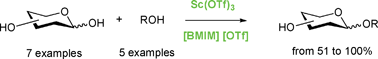Vous êtes ici :
- Unité de recherche
- BioCIS
Publication2
1. Hirao cross-coupling reaction as efficient tool to build non-natural C2-phosphonylated sugars, Monasson, O.; Malinowski, M.; Lubin-Germain, N.; Ferry, A.; Synthesis, 2022, ASAP article
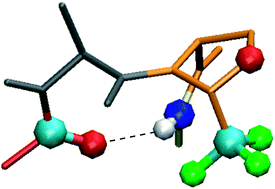
2. Introducing the chiral constrained α-trifluoromethylalanine in aib foldamers to control, quantify and assign the helical screw-sense, Bodero, L., Guitot, K., Lensen, N., Lequin, O., Brigaud, T., Ongeri, S., Chaume, G. Chemistry - A European Journal, 2022, ASAP article

3. (R)-α-Trifluoromethylalanine as a 19F NMR Probe for the Monitoring of Protease Digestion of Peptides. Devillers, E.; Chelain, E.; Dalvit, C.; Brigaud, T.; Pytkowicz, J. ChemBioChem, 2022, ASAP article

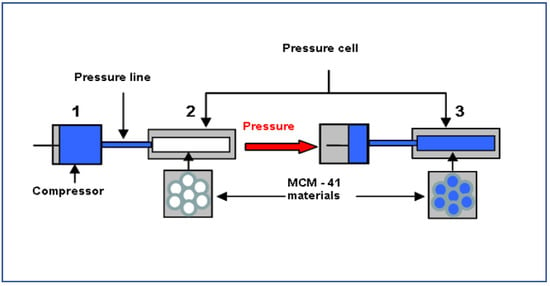
1. Copper and Nickel Nanoparticles Prepared by Thermal Treatment of Their Respective Cations Confined in Nanopores through High-Pressure Synthesis. Brodie-Linder,N.; Deschamps, J.; Bombled, M.; Pasternak, N.; Audonnet, F.; Beaunier, P.; Alba-Simionesco, C. Appl. Nano 2021, 278-288
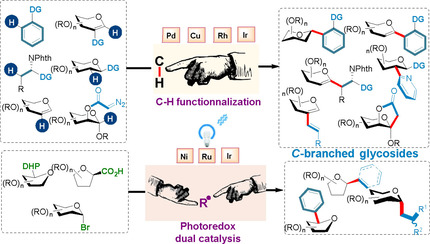
2. Emerging Organometallic Methods for the Synthesis of C-Branched (Hetero)aryl, Alkenyl, and Alkyl Glycosides: C-H Functionalization and Dual Photoredox Approaches, Ghouilem, J.; de Robichon, M.; Le Bideau, F.; Ferry, A.; Messaoudi, S; Chemistry - A European Journal, 2021, 27(2), pp. 491–511
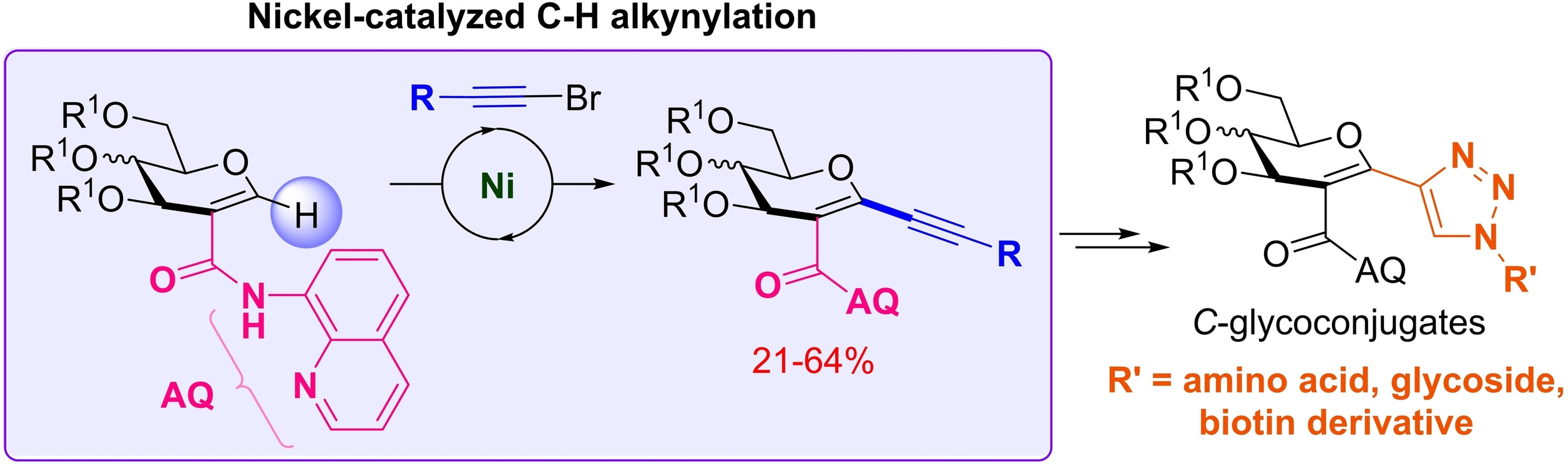
3. Directed Nickel-Catalyzed Pseudo-Anomeric C−H Alkynylation of Glycals as an Approach towards C-Glycoconjugate Synthesis. de Robichon, M.; Branquet, D.; Uziel, J.; Lubin-Germain, N.; Ferry, A. Adv. Synth. Catal. 2021 ASAP article.
4. Identification and characteristics of fusion peptides derived from enveloped viruses C. Lozada, T. M. A. Barlow, S. Gonzalez, N. Lubin-Germain, S. Ballet Front. Chem. 2021, 9:689006
5. Synthesis of enantiopure α-Tfm-proline and α-Tfm-pipecolic acid from oxazolo-pyrrolidines and -piperidines. C. A. Sanchez, C. Gadais, S. Diarra, A. Bordessa, N. Lensen, E. Chelain and T. Brigaud, Org. Biomol. Chem., 2021, 6771-6775
6. Synthesis of the Fungal Metabolite YWA1 and Related Constructs as Tools to Study MelLec-Mediated Immune Response to Aspergillus Infections. Piras, M.; Patruno, I.; Nikolakopoulou, C.; Willment, J. A.; Sloan, N. L.; Zanato, C.; Brown, G. D.; Zanda, M. J. Org. Chem. 2021, 6044–6055

7. Glycosamine Derivatives through Metal-Catalyzed C−N Bond Formation on Protected and Unprotected 2-Iodoglycals. Malinowski, M.; Banoun, C.; de Robichon, M.; Lubin-Germain, N.; Ferry, A. Eur. J. Org. Chem. 2021, 1521–1524.

8. Enantiopure 5-CF3–Proline: Synthesis, Incorporation in Peptides, and Tuning of the Peptide Bond Geometry. C. A. Sanchez, C. Gadais, C.;Chaume, G.; Girard, S.; Chelain, E.; Brigaud, T. Org. Lett. 2021, 382-387

1. Mild Palladium‐Catalyzed Cyanation of Unprotected 2‐Iodoglycals in Aqueous Media as Versatile Tool to Access Diverse C2‐Glycoanalogues. Malinowski, M.; Tran, T. V.; de Robichon, M.; Lubin-Germain, N.; Ferry, A. Adv. Synth. Catal. 2020, 362, 1184-1189.

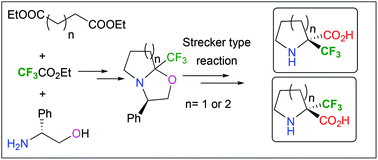
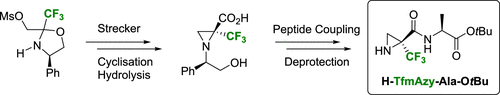
2. Enantiopure α-Trifluoromethylated Aziridine-2-carboxylic Acid (α-TfmAzy): Synthesis and Peptide Coupling. Ouerfelli, O.; Simon, J.; Chelain, E.; Pytkowicz, J.; Besbes, R.; Brigaud, T. Org. Lett. 2020, 22, 2946-2949.
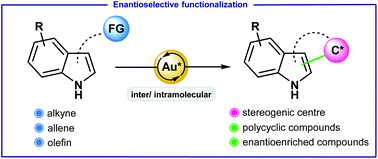
3. Gold-catalyzed enantioselective functionalization of indoles. Milcendeau, P.; Sabat, N.; Ferry, A. ; Guinchard, X. Org. Biomol. Chem. 2020, 18, 6006-6017.
4. Identification of NPB, NPW and Their Receptor in the Rat Heart. Pandey, S.; Tuma, Z.; Peroni, E.; Monasson, O.; Papini, A. M.; Dvorakova, M. C. Int. J. Mol. Sci. 2020, 21, 7827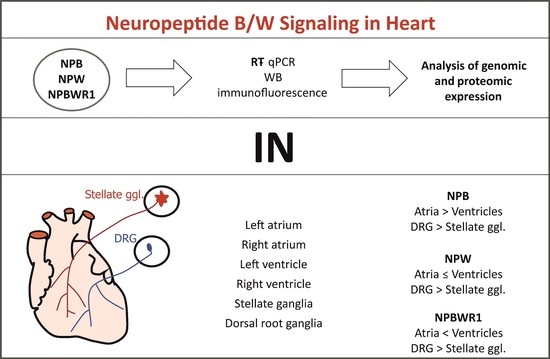
5. A Multiple N-Glucosylated Peptide Epitope Efficiently Detecting Antibodies in Multiple Sclerosis. Nuti, F., Fernandez, FR., Sabatino, G., Peroni, E., Mulinacci, B., Paolini, I., Di Pisa, M., Tiberi, C., Lolli, F., Petruzzo, M., Lanzillo, R., Brescia Morra, V., Rovero, P., Papini A. M. Brain Sci. 2020, 10, 453.
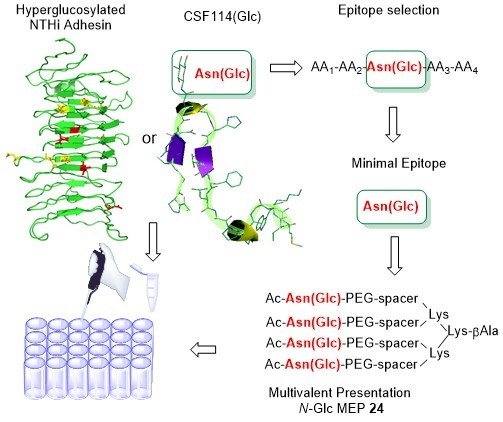
6. Hyperglucosylated adhesin-derived peptides as antigenic probes in multiple sclerosis: Structure optimization and immunological evaluation. Mazzoleni, A.,Real-Fernandez, F., Larregola, M., Nuti, F., Lequin, O., Papini, AM., Mallet, JM., Rovero, P. J. Pept. Sci. 2020, 26, e3281.
7. Direct Synthesis of Mesoporous Organosilica and Proof-of-Concept Applications in Lysozyme Adsorption and Supported Catalysis. Osta, O., Bombled, M., Partouche, D., Gallier, F., Lubin-Germain, N., Brodie-Linder, N., Alba-Simionesco, C. ACS Omega 2020, 5, 18842-18848.

8. Synthesis and antitumor activities investigation of a C-nucleoside analogue of ribavirin. Sabat, N., Migianu-Griffoni, E., Tudela, T., Lecouvey, M., Kellouche, S., Carreiras, F., Gallier, F., Uziel, J., Lubin-Germain, N. Eur. J. Med. Chem. 2020, 188, 112009

9.« Les pigments verts en Égypte au début de la Troisième Période intermédiaire : de l’objet à l’artisan ». Lucile Brunel-Duverger, Yvan Coquinot, Nancy Brodie-Linder et Sandrine Pagès-Camagna Techne 50 2020-2
1. Studies of membranotropic and fusogenic activity of two putative HCV fusion peptides. Gonzalez, S.; Gallier, F.; Kellouche, S.; Carreiras, F.; Novellino, E.; Carotenuto, A.; Chassaing, G.; Rovero, P.; Uziel, J.; Lubin-Germain, N. Biochim Biophys Acta Biomembr. 2019, 1861, 50–61.

2. A photochromic azobenzene peptidomimetic of a β-turn model peptide structure as a conformational switch. Papini, A. M.; Nuti, F.; Gellini, C.; Larregola, M.; Squillantini, L.; Chelli, R.; Salvi, P. R.; Lequin, O.; Pietraperzia, G. Front. Chem. - Supramolecular Chemistry, 2019, 7:180.
3. “CO” as a Carbon Bridge to Build Complex C2-Branched Glycosides Using a Palladium-Catalyzed Carbonylative Suzuki–Miyaura Reaction from 2-Iodoglycals. de Robichon, M.; Bordessa, A.; Lubin-Germain, N.; Ferry, A. J. Org. Chem. 2019, 84, 3328-3339.
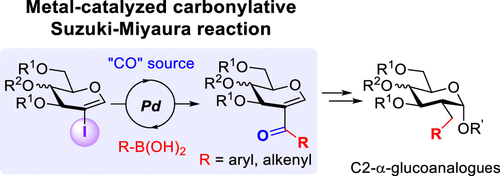
4. Access to C-aryl/alkenylglycosides by directed Pd-catalyzed C–H functionalisation of the anomeric position in glycal-type substrates. de Robichon, M.; Bordessa, A.; Malinowski, M.; Uziel, J.; Lubin-Germain, N.; Ferry, A. Chem. Commun. 2019, 55, 11806-11808.
5. CF2H as hydrogen bond donor group for the fine tuning of peptide bond geometry with difluoromethylated pseudoprolines. Malquin, N.; Rahgoshay, K.; Lensen, N.; Chaume, G.; Miclet, E.; Brigaud, T. Chem. Commun. 2019, 55, 12487-12490.
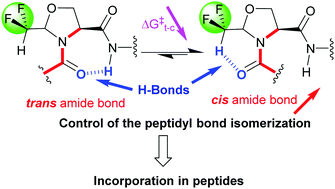
6. Trifluoromethylated Proline Surrogates as Part of "Pro-Pro" Turn-Inducing Templates. Gadais, C.; Van holsbeeck, K.; Moors, S. L. C.; Buyst, D.; Feher, K.; Van Hecke, K.; Tourwe, D.; Brigaud, T.; Martin, C.; De Proft, F.; Pytkowicz, J.; Martins, J. C.; Chaume, G.; Ballet, S. ChemBioChem 2019, 20, 2513-2518.

7. Photochemical Origin of the Darkening of Copper Acetate and Resinate Pigments in Historical Paintings M. Alter, L. Binet, N.Touati, N. Lubin-Germain, A.S.Le Hô, F. Mirambet, D.Gourier Inorg. Chem. 2019, 58, 19, 13115–13128.

8. Evaluation of the potential of a new ribavirin analog impairing the dissemination of ovarian cancer cells A. Wambecke, C. Laurent-Issartel, J. Leroy-Dudal, F. Giffard, F. Cosson, N.Lubin-Germain, J. Uziel, S. Kellouche, F. Carreiras Plos One 2019, 14 (12), e02225860.
9. The contribution of non-invasive and non-destructive techniques to the understanding of the 21st Dynasty Egyptian Yellow Coffins complex stratigraphy: Case of study of E 20043 from the Louvre Museum. Brunel-Duverger L., Laval E., Lemasson Q., Brodie-Linder N., Pages-Camagna, S. The European Physical Journal Plus 2019, 134, 257
1. Orthogonal 19F-labeling for solid-state NMR reveals the conformation and orientation of short peptaibols in membranes. Grage, S. L.; Kara, S.; Bordessa, A.; Doan, V.; Rizzolo, F.; Putzu, M.; Kubař, T.; Papini, A. M.; Chaume, G.; Brigaud, T.; Afonin, S.; Ulrich, A. S. Chem. Eur. J. 2018, 24, 4328-4335

2. Probing the outstanding local hydrophobicity increase of peptide sequences induced by trifluoromethylated amino acids incorporation. Gadais, C.; Devillers, E.; Gasparik, V.; Chelain, E.; Pytkowicz, J.; Brigaud, T. ChemBioChem 2018, 19, 1026–1030.

3.Trifluoromethylated proline analogues as efficient tools to enhance the hydrophobicity and to promote passive diffusion transport of the L-prolyl-L-leucyl glycinamide (PLG) tripeptide. Oliver, M.; Gadais, C.; Garcia-Pindado, J.; Teixido, M.; Lensen, N.; Chaume, G.; Brigaud, T. RSC Adv. 2018, 8, 14597-14602.

4. Lipase-Catalyzed Amidation of Carboxylic Acid and Amines. Manova; D.; Gallier, F.; Tak-Tak, L.; Yotava, L.; Lubin-Germain, N. Tetrahedron Lett 2018, 59, 2086-2090.

5. Synthesis of C-pyrimidyl nucleosides starting from alkynyl ribofuranosides. Legrave, G.; Ait Youcef, R.; Afonso, D.; Ferry, A.; Uziel, J.; Lubin-Germain, N. Carbohydr. Res. 2018, 462, 50-55.

6. Antibodies to post-translationally modified mitochondrial peptide PDC-E2(167-184) in type 1 diabetes. Nuti, F.; Gallo, A.; Real-Fernandez, F.; Crulli, M.; Rentier, C.; Piarulli, F.; Peroni, E.; Rossi, G.; Traldi, P.; Rovero, P.; Lapolla, A.; Papini, A.M. Arch. Biochem. Biophys. 2018, 659, 66-74.
7. Stereoselective synthesis of 4-hydroxymethyl-1,3-oxazolidin-2-one derivatives from novel 2-hydroxymethylaziridines. Ouerfelli, O.; Ali Tabarki, M.; Pytkowicz, J.; Arfaoui, Y.; Brigaud, T.; Besbes, R. Synth. Commun. 2018, 48, 2242-2252

1. Synthesis of C-Ribosyl-1,2,3-triazolyl Carboxamides. Solarte, C.; Dos Santos, M.; Gonzalez, S.; Miranda, L. S. M.; Guillot, R.; Ferry, A.; Gallier, F.; Uziel, J.; Lubin-Germain, N. Synthesis 2017, 49, 1993-2002.

2. Enantioselective Access to Robinson Annulation Products & to Michael Adducts Precursors: Where Do We Stand After 80 Years? Gallier, F.; Martel, A.; Dujardin, G. Angew. Chem. Int. Ed. 2017, 56, 12424–12458.
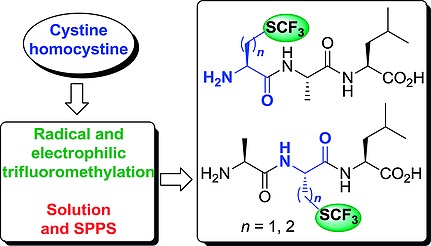
3.Tailored Approaches towards the Synthesis of L-S-(Trifluoromethyl)cysteine- and L-Trifluoromethionine-Containing Peptides. Gadais, C.; Saraiva-Rosa, N.; Chelain, E.; Pytkowicz, J.; Brigaud, T. Eur. J. Org. Chem. 2017, 246-251.
4. Structure-Activity Relationship Studies, SPR Affinity Characterization, and Conformational Analysis of Peptides That Mimic the HNK-1 Carbohydrate Epitope. Ieronymaki, M ; Nuti, F ; Brancaccio, D ; Rossi, G ; Real-Fernandez, F ; Cao, YH ; Monasson, O ; Larregola, M ; Peroni, E ; Uziel, J; Sabatino, G ; Novellino, E ; Carotenuto, A ; Papini, AM ; Rovero, P. ChemMedChem 2017, 12, 751-759.
5. Synthesis of Ribavirin 2’-Me-C-nucleosides analogues. Cosson, F.; Faroux, A.; Baltaze, J.P.; Farjon, J.; Guillot, R.; Uziel, J.; Lubin-Germain, N. Beilstein J. Org. Chem. 2017, 13, 755-761.
6. Structural Behavior of the Peptaibol Harzianin HK VI in a DMPC Bilayer: Insights from MD Simulations. Putzu, M.; Kara, S.; Afonin, S.; Grage, S. L.; Bordessa, A.; Chaume, G.; Brigaud, T.; Ulrich, A. S.; Kubar, T. Biophys. J. 2017, 112, 2602-2614.
7. Homochiral versus Heterochiral Trifluoromethylated Pseudoproline Containing Dipeptides: A Powerful Tool to Switch the Prolyl-Amide Bond Conformation. Chaume, G.; Simon, J.; Lensen, N.; Pytkowicz, J.; Brigaud, T.; Miclet, E. J. Org. Chem. 2017, 82, 13602-13608.

1. Incorporation of Trifluoromethylated Proline and Surrogates into Peptides: Application to the Synthesis of Fluorinated Analogues of the Neuroprotective Glycine-Proline-Glutamate (GPE) Tripeptide. Simon, J.; Pytkowicz, J.; Lensen, N.; Chaume, G.; Brigaud, T. J. Org. Chem. 2016, 81, 5381-5392.

2. Synthesis of protected enantiopure (R) and (S)-α-trifluoromethylalanine containing dipeptide building blocks ready to use for solid phase peptide synthesis. Devillers, E.; Pytkowicz, J.; Chelain, E.; Brigaud, T. Amino Acids 2016, 48, 1457-1468.
3. Epitope mapping of anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in a mouse model of multiple sclerosis: microwave-assisted synthesis of the peptide antigens and ELISA screening. Pacini, G.; Ieronymaki, M.; Nuti, F.; Sabatina, G.; Larregola, M.; Aharoni, R.; Papini, A. M.; Rovero, P. J. Pept. Sci. 2016, 22, 52-58.
4. Functionalization of 2H-1,2,3-Triazole C-Nucleoside Template via N2 Selective Arylation. Lopes, A. B.; Wagner, P.; Alves de Souza, R. O. M.; Lubin Germain, N.; Uziel, J.; Bourguignon, J.-J.; Schmitt, M.; Miranda, L. S. M. J. Org. Chem. 2016, 81, 4540-4549.

5. Metal Promoted Diels-Alder Reactions. Gallier, F. Current Organic Chemistry, 2016, 20(21), 2222-2253.

6. The weight of flash chromatography: a tool to predict the mass intensity from thin-layer chromatography. Pessel, F.; Augé, J.; Billault, I.; Scherrmann, M-C. Beilstein J. Org. Chem. 2016, 12, 2351-2357.
7. Access to Complex C2-Branched Glycoconjugates via Palladium-Catalyzed Aminocarbonylation Reaction of 2-Iodoglycals. Bordessa, A.; Ferry, A.; Lubin-Germain, N. J. Org. Chem. 2016, 81 (24), 12459–12465.

1.Straightforward Synthesis of Novel Enantiopure α-Trifluoromethylated Azetidine 2-Carboxylic Acid and Homoserines. Lensen, N.; Marais, J.; Brigaud, T. Org. Lett. 2015, 17, 342-345

2.Synthesis of Enantiopure trans-2,5-Disubstituted Trifluoromethylpyrrolidines and (2S,5R)‑5-Trifluoromethylproline. Lubin, H.; Pytkowicz, J.; Chaume, G.; Sizun-Thomé, G.; Brigaud, T. J. Org. Chem. 2015, 80, 2700-2708

3. Methionine sulfoxide- and sulfone-containing peptide synthesis: management of a relevant post-translational modification in proteins. Rentier, C.; Monasson, O.; Nuti, F.; Rovero, P.; Sabatino, G.; Papini, A-M. Chemistry Today 2015, 33, 32-36.
4. Synthesis of diastereomerically pure Lys(Nε-lipoyl) building blocks and their use in Fmoc/tBu solid phase synthesis of lipoyl-containing peptides for diagnosis of primary biliary cirrhosis. Rentier, C.; Pacini, G.; Nuti, F.; Peroni, E.; Rovero, P.; Papini, A-M. J. Pept. Sci. 2015, 21, 408-414.
5. Antibody Recognition in Multiple Sclerosis and Rett Syndrome Using a Collection of Linear and Cyclic N-glucosylated Antigenic Probes. Real Fernández, F.; Di Pisa, M.; Rossi, G.; Auberger, N.; Lequin, O.; Larregola, M.; Benchohra, A.; Mansuy, C.; Chassaing, G.; Lolli, F.; Hayek, J.; Lavielle, S.; Rovero, P.; Mallet, J-M.; Papini, A-M. Biopolymers 2015, 104, 560-576
6. Use of a (R)-α-Trifluoromethylalanine Containing Short Peptide in the Inhibition of Amyloid Peptide Fibrillation. Botz, A.; Gasparik, V.; Devillers, E.; Hoffmann, A. R. F.; Caillon, L.; Chelain, E.; Lequin, O.; Brigaud, T.; Khemtemourian, L. Biopolymers 2015, 104, 601-610
7. Role of lipoylation of the immunodominant epitope of pyruvate dehydrogenase complex: toward a peptide-based diagnostic immunoassay for primary biliary cirrhosis. Pacini, G.; Carotenuto, A.; Rentier, C.; Nuti, F.; Real-Fernandez, F.; Brancaccio, D.; Sabatino, G.; Larregola, M.; Peroni, E.; Migliorini, P.; Novellino, E.; Battezzati, P. M.; Selmi, C.; Papini, A. M.; Rovero, P. J. Med. Chem. 2015, 58, 6619-6629

8. Blanching of paint and varnish layers in easel paintings: contribution to the understanding of the alteration. Genty-Vincent, A.; Eveno, M.; Nowik, W.; Bastian, G.; Ravaud, E.; Cabillic, I.; Uziel, J.; Lubin-Germain, N.; Menu, M. Appl. Phys. A-Mater. 2015, 121, 779-788.
9. Interactions between Human Antibodies and Synthetic Conformational Peptide Epitopes: Innovative Approach for Electrochemical Detection of Biomarkers of Multiple Sclerosis at Platinum Electrodes. Bellagha-Chenchah, W.; Sella, C.; Real-Fernandez, F.; Peroni, E.; Lolli, F.; Amatore, C.; Thouin, L.; Papini, A. M. Electrochim. Acta 2015, 176, 1239-1247.
10. Synthesis of diastereomerically pure Lys(Nε-lipoyl) building blocks and their use in Fmoc/tBu solid phase synthesis of lipoyl-containing peptides for diagnosis of primary biliary cirrhosis. Rentier C., Pacini G., Nuti F., Peroni E., Rovero P., Papini A.M. J. Pept. Sci. 2015, 21, 408-414.
11. From the capillary condensation to the glass transition of a confined molecular liquid: Case of toluene. Audonnet, F.; Brodie-Linder, N.; Morineau, D.; Frick, B. J. Non-Cryst. Solids 2015, 407, 262-269.
12. Characterizing pigments with hyperspectral imaging variable false-color composites. Hayem-Ghez, A.; Ravaud, E.; Boust, C.; Bastian, G.; Menu, M.; Brodie-Linder, N. Appl. Phys. A 2015, 121, 939-947.
1. Copper(II) SBA-15: A reusable catalyst for azide-alkyne cycloaddition, Jlalia, I.; Gallier, F.; Brodie-Linder, N.; Uziel, J.; Auge, J.; Lubin-Germain, N. J. Mol. Catal. A: Chem. 2014, 393, 56-61.

2. Lubineau’s green synthons, Augé, J.; Lubin-Germain, N. Carbohydr. Chem. 2014, 40, 11-30.
3. Glaser oxidative coupling on peptides: Stabilization of β-turn structure via a 1,3-butadiyne constraint. Auberger, N.; Di Pisa, M.; Larregola, M.; Chassaing, G.; Peroni, E.; Lavielle, S.; Papini, A. -M.; Lequin, O.; Mallet, J. -M. Bioorg. Med. Chem. 2014, 22, 6924–6932.

4. 1,4-Disubstituted-[1,2,3]triazolyl-Containing Analogues of MT-II: Design, Synthesis, Conformational Analysis, and Biological Activity. Testa, C.; Scrima, M.; Grimaldi, M.; D'Ursi, A. M.; Dirain, M. L.; Lubin-Germain, N.; Singh, A.; Haskell-Luevano, C.; Chorev, M.; Rovero, P.; Papini, A. M. J. Med. Chem. 2014, 57, 9424-9434
 .
.
5. New highlights on degradation process of verdigris from easel paintings. Santoro, C.; Zarkout, K.; Le Hô, A.S.; Mirambet, F.; Gourier, D.; Binet, L.; Pagès-Camagna, S.; Reguer, S.; Mirabaud, S.; Le Du, Y.; Griesmar, P.; Lubin-Germain, N.; Menu, M. Applied Physics A 2014. 114, 637-645.
1.Incorporation of CF3−Pseudoprolines into Peptides: A Methodological Study. Chaume, G.; Simon, J.; Caupène, C.; Lensen, N.; Miclet, E.; Brigaud, T. J. Org. Chem. 2013, 78, 10144-10153.

2. Effects of enantiopure (S)-a-trifluoromethyl proline containing MIF-1’s analogue on stress-induced analgesia. Bocheva, A.; Nocheva, H.; Jlalia, I.; Lensen, N.; Chaume, G.; Brigaud, T. Med. Chem. 2013, 3, 206-209.
3.Synthesis of a MIF-1 analogue containing Enantiopure (S)-α-Trifluoromethyl Proline and Biological Evaluation on Nociception. Jlalia, I.; Lensen, N.; Chaume, G.; Dzhambazova, E.; Astasidi, L.; Hadjiolova, R.; Bocheva, A.; Brigaud, T. Eur. J. Med. Chem. 2013, 62, 122-129.

4. Conformational properties of peptides incorporating a fluorinated pseudoproline residue. Chaume, G.; Feytens, D.; Chassaing, G.; Lavielle, S.; Brigaud, T.; Miclet, E. New J. Chem. 2013, 37, 1336-1342.
5. Crystallization-Induced Dynamic Resolution of Fox Chiral Auxiliary and Application to the Diastereoselective Electrophilic Fluorination of Amide Enolates. Lubin, H.; Dupuis, C.; Pytkowicz, J.; Brigaud, T. J. Org. Chem. 2013, 78, 3487-3492.

6. Alpha actinin is specifically recognized by Multiple Sclerosis autoantibodies isolated using an N-glucosylated peptide epitope. Pandey, S.; Dioni, I.; Lambardi, D.; Real-Fernandez, F.; Peroni, E.; Pacini, G.; Lolli, F.; Seraglia, R.; Papini, A. M.; Rovero, P. Mol. Cell. Proteomics 2013, 12, 277–282.
7. Evaluation of new immunological targets in neuromyelitis optica. Chanson, J.-B.; Paolini, I.; Collongues, N.; Alcaro, M. C.; Blanc, F.; Barbetti, F.; Fleury, M.; Peroni, E.; Rovero, P.; Rudolf, G.; Lolli, F.; Trifilieff, É.; Papini, A.-M.; De Seze, J. J. Pept. Sci. 2013, 19, 25–32.
8. Method to create a hydrophilic environment within hydrophobic nanostructures, Brodie-Linder, N.; Deschamps, J.; Audonnet, F.; Alba-Simionesco, C. Micropor. Mesopor. Mat. 2013, 179, 17–21.
9. Total synthesis of high loading capacity PEG-based supports: evaluation and improvement of the process by use of ultrafiltration and PEG as a solvent, Turgis, R.; Billault, I.; Acherar, S.; Augé, J.; Scherrmann, M.-C. Green Chem. 2013, 15, 1016–1029.
1. Local control of the cis-trans isomerization and backbone dihedral angles in peptides using trifluoromethylated pseudoprolines, Feytens, D.; Chaume, G.; Chassaing, G.; Lavielle, S.; Brigaud, T.; Byun, B. J.; Kang, Y. K.; Miclet, E. J. Phys. Chem. B 2012, 116, 4069-4079.

2. Determination of the global material economy (GME) of synthesis sequences-a green chemistry metric to evaluate the greenness of products, Augé, J.; Scherrmann, M.-C. New J. Chem, 2012, 36, 1091-1098.
3. Straightforward glycosylation of alcohols and amino acids mediated by ionic liquid, Monasson, O.; Sizun-Thomé, G.; Lubin-Germain, N.; Uziel, J.; Augé, J. Carbohydr. Res. 2012, 352, 202-205
4. 2-Trifluoromethyl-2-methyl-4-phenyloxazolidine: A new chiral auxiliary for highly diastereoselective enolate alkylation, Tessier, A.; Pytkowicz, J.; Brigaud, T. J Fluorine Chem. 2012, 134, 122-127

5. Highly Diastereoselective Synthesis of Enantiopure β-Trifluoromethyl β-Amino Alcohols from Chiral Trifluoromethyl Oxazolidines (Fox), Simon, J.; Chelain, E.; Brigaud, T. Org. Lett., 2012, 14 (2), 604–607

6. Glycopeptide-Based Antibody Detection in Multiple Sclerosis by Surface Plasmon Resonance, Real-Fernandez, F.; Passalacqua, I.; Peroni, E.; Chelli, M.; Lolli, F.; Papini, A. M.; Rovero, P. Sensors 2012, 12, 5596-5607.
7. Designed glucopeptides mimetics of myelin protein epitopes as synthetic probes for the detection of autoantibodies, biomarkers of multiple sclerosis. Pandey, S.; Alcaro, M. C.; Scrima, M.; Peroni, E.; Paolini, I.; Di Marino, S.; Barbetti, F.; Carotenuto, A.; Novellino, E.; Papini, A. M.; D’Ursi, A. M.; Rovero, P. J. Med. Chem. 2012, 55, 10437–10447.

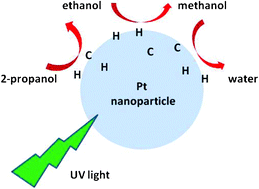
8. Alcohol to water catalyzed by Pt nanoparticles: an experimental and computational approach, Dehouche, F.; Archirel, P.; Remita, H.; Brodie-Linder, N.; Traverse, A.. RSC Advances 2012, 2, 6686.
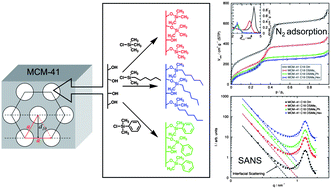
9. Wall thickness determination of hydrophobically functionalized MCM-41 materials, Schoeffel, M.; Brodie-Linder, N.; Audonnet, F.; Alba-Simionesco, C. J. Mater. Chem. 2012, 22, 557–567.
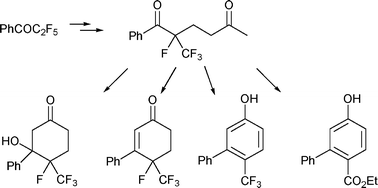
1. Synthesis of trifluoromethyl cyclohexyl, cyclohexenyl and aryl compounds via stepwise Robinson annulation, Massicot, F.; Iriarte, A. M.; Brigaud, T.; Lebrun, A.; Portella, C. Org. Biomol. Chem. 2011, 2, 600-603.
2. Concise Synthesis of Enantiopure (S)- and (R)-alpha-Trifluoromethyl Aspartic Acid and alpha-Trifluoromethyl Serine from Chiral Trifluoromethyl Oxazolidines (Fox) via the Strecker-type Reaction, Simon, J.; Nguyen, T. T.; Chelain, E.; Lensen, N.; Pytkowicz, J.; Chaume, G.; Brigaud, T. Tetrahedron : Asymmetry 2011, 22, 309-314.

3. IgG and IgM antibodies to the refolded MOG(1-125) extracellular domain in humans, Gori, F.; Mulinacci, B.; Massai, L.; Avolio, C.; Caragnano, M.; Peroni, E.; Lori, S.; Chelli, M.; Papini, A-M.; Rovero, P. J. Neuroimmunol. 2011, 233, 216-220.
4. Titan Based Hybrid Organic-Inorganic Gels Comprising Carbohydrate Moiety, Danalev, D. L.; Lubin-Germain, N.; Serfaty, S.; Le Huerou, J-Y.; Auge, J.; Uziel, J.; Griesmar, P. Phosphorus Sulfur 2011, 186, 2216-2225.

5. La chimie verte pour un avenir plus rose, Augé, J.; Scherrmann, M.-C. Découverte 2011, 377, 28-37.
1. Highly Diastereoselective alfa-Hydroxylation of Fox Chiral Auxiliary-Based Amide Enolates with Molecular Oxygen, Lubin, H. ; Tessier, A. ; Chaume, G. ; Pytkowicz, J. ; Brigaud, T. Org. Lett. 2010, 12, 1496-1499.

2. Synthesis of 2-Trifluoromethyl-1,3-oxazolidines as Hydrolytically Stable Pseudoprolines, Chaume, G. ; Barbeau, O. ; Lesot, P. ; Brigaud, T. J. Org. Chem. 2010, 75, 4135-4145.

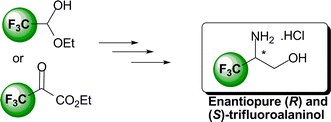
3. Straightforward synthesis of enantiopure (R)- and (S)-trifluoroalaninol, Pytkowicz, J. ; Stephany, O. ; Marinkovic, S. ; Inagaki, S. ; Brigaud, T. Org. Biomol. Chem. 2010, 8, 4540-4542
.
4. Posttranslationally modified peptides efficiently mimicking neoantigens : a challenge for theragnostics of autoimmune diseases, Nuti, F.; Peroni, E.; Real-Fernandez, F.; Bonache, M. A.; Le Chevalier-Isaad, A.; Chelli, M.; Lubin-Germain, N.; Uziel, J.; Rovero, P.; Lolli, F.; Papini, A. M. Biopolymers 2010, 94, 791-799.
5. Synthesis of alkynes and alkynyl iodides bearing a protected amino alcohol moiety as functionalized amino acids precursors, Ayed, C.; Picard, J.; Lubin-Germain, N.; Uziel, J.; Auge, J. Sci. China Chem. 2010, 53, 1921-1926.
6. Indium-mediated alkynylation of sugars : synthesis of C-glycosyl compounds bearing a protected amino alcohol moiety, Ayed, C.; Palmier, S.; Lubin-Germain, N.; Uziel, J.; Auge, J. Carbohydr. Res. 2010, 345, 2566-2570.

1. Asymmetric aldol reactions using chiral CF3-Oxazolidines (Fox) as chiral auxiliary,Tessier, A. ; Pytkowicz, J. ; Brigaud, T. J. Fluorine Chem., 2009, 130, 1140-1144

2. Iodocyclization of Chiral CF3-Allylmorpholinones : A Versatile Strategy for the Synthesis of Enantiopure alfa-Tfm-Prolines and alfa-Tfm-Dihydroxyprolines, Caupène, C. ; Chaume, G. ; Ricard, L. ; Brigaud, T. Org. Lett. 2009, 11, 209-212

3. Convenient Synthesis of N-Terminal Tfm-Dipeptides from Unprotected Enantiopure alfa-Tfm-Proline and alfa-Tfm-Alanine, Chaume, G. ; Lensen, N. ; Caupène, C. ; Brigaud, T. Eur. J. Org. Chem. 2009, 33, 5717-5724

4. Huisgen Cycloaddition Reaction of C-Alkynyl Ribosides under Micellar Catalysis : Synthesis of Ribavirin Analogues, Youcef, R. A. ; Dos Santos, M. ; Roussel, S. ; Baltaze, J. -P. ; Lubin-Germain, N. ; Uziel, J. J. Org. Chem. 2009, 74, 4318-4323

5. Ionic liquid promoted atom economic glycosylation under Lewis acid catalysis, Augé, J. ; Sizun, G. Green Chem. 2009, 11, 1179-1183
1. Umpolung reactivity of difluoroenol silyl ethers with amines and amino alcohols. Application to the synthesis of enantiopure alfa-difluoromethyl amines and amino acids, Huguenot, F. ; Billac, A. ; Brigaud, T. ; Portella, C. J. Org. Chem. 2008, 73, 2564-2569

2. Concise access to enantiopure (S)- and (R)-α-trifluoromethyl pyroglutamic acids from ethyl trifluoropyruvate-based chiral CF3-oxazolidines (Fox), Chaume, G. ; Van Severen, M. -C. ; Ricard, L. ; Brigaud, T. J. Fluorine Chem. 2008, 129, 1104-1109

3. Fluorine••• and π•••Alcali Metal Interactions control in the Stereoselective Amide Enolates Alkylation using Fluorinated Oxazolidines (Fox) as Chiral Auxiliary : An Experimental and Theoretical Study, Sini, G. ; Tessier, A. ; Pytkowicz, J. ; Brigaud, T. Chem. Eur. J. 2008, 14, 3363-3370
4. Highly diastereoselective synthetic route to enantiopure β2-amino acids and γ-amino alcohols using a fluorinated oxazolidine (Fox) as chiral auxiliary, Tessier, A. ; Lahmar, N. ; Pytkowicz, J. ; Brigaud, T. J. Org. Chem. 2008, 73, 3970-3973

5. Direct C-glycosylation by indium-mediated alkynylation on sugar anomeric position, Lubin-Germain, N. ; Baltaze, J. -P. ; Coste, A. ; Hallonet, A. ; Lauréano, H. ; Legrave, G. ; Uziel, J. ; Augé, J. Org. Lett. 2008, 10, 725-728

6. A new rationale of reaction metrics for green chemistry. Mathematical expression of the environmental impact chemical processes, Augé, J. Green Chem. 2008, 10, 225-231
7. Ferrocenyl Glycopeptides as Electrochemical Probes to Detect Autoantibodies in Multiple Sclerosis Patients’ Sera, Real Fernández, F. ; Chamois-Colson, A. ; Bayardon, J. ; Nuti, F. ; Peroni, E. ; Meunier-Prest, R. ; Lolli, F. ; Chelli, M. ; Darcel, C. ; Jugé, S. ; Papini, A. M. Pept. Science 2008, 90, 488-495
8. Design glycopeptides with different β-turn types as synthetic probes for the detection of autoantibodies of multiple sclerosis, Carotenuto, A. ; Alcaro, A. C. ; Saviello, M. R. ; Peroni, E. ; Nuti, F. ; Papini, A. M. ; Novellino, E. ; Rovero, P. J. Med. Chem. 2008, 51, 5304-5309