You are here :
- Unité de recherche
- BioCIS
- Home
- Research topics
- Peptidomimetics
Fluorinated Peptides
One of the limitations to the use of peptides as therapeutic agents is their rapid degradation by peptidases as well as their low lipophilicity. Our laboratory is interested in the development of efficient peptide coupling reactions that allow the incorporation of enantiopure α-trifluoromethylated amino acids into peptide chains. Their use is of great interest due to the unique properties of the fluorine atom. Their incorporation into peptide chains is promising due to conformational constraints, electronic effects and increased hydrophobicity induced by fluorinated groups. Thus an increased metabolic stability with respect to proteases and original three-dimensional structures are expected. The local increase in the hydrophobic character can make it possible to facilitate the passage of the blood-brain barrier. Moreover, these fluorinated peptides have a strong analytical potential as an NMR probe (19F) for the elucidation of structures, the understanding of biological mechanisms and the pharmacokinetic studies of these non-proteogenic peptides.
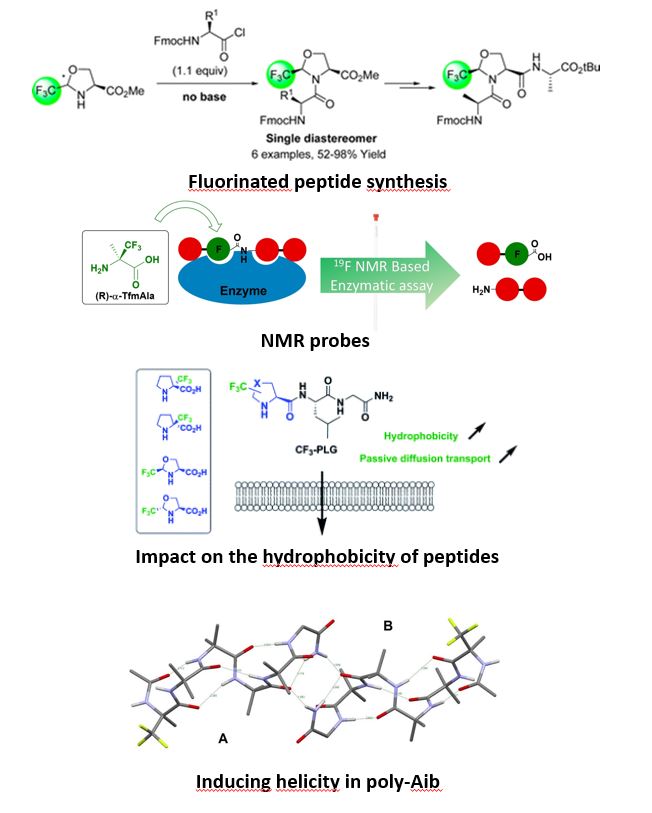
We have recently developed an efficient method for the synthesis of N-terminal dipeptides from unprotected α-Tfm-Alanine and α-Tfm-Proline using classical HOBt, EDCI peptide coupling conditions. The synthesis of C-terminal dipeptides is much more difficult due to the strong deactivation of nitrogen by the CF3 group. A methodological study carried out using chiral trifluoromethylated pseudoprolines made it possible to carry out the coupling reaction with activated amino acids in the form of acyl chlorides. The optimization of the reaction conditions is in progress in the laboratory and they are applied to the synthesis of various peptides of interest.
- Selected publications
-
1. Introducing the chiral constrained α-trifluoromethylalanine in aib foldamers to control, quantify and assign the helical screw-sense, Bodero, L., Guitot, K., Lensen, N., Lequin, O., Brigaud, T., Ongeri, S., Chaume, G. Chemistry - A European Journal, 2022, ASAP article
2. (R)-α-Trifluoromethylalanine as a 19F NMR Probe for the Monitoring of Protease Digestion of Peptides. Devillers, E.; Chelain, E.; Dalvit, C.; Brigaud, T.; Pytkowicz, J., ChemBioChem 2022,23, e202100470
3. Enantiopure 5-CF3–Proline: Synthesis, Incorporation in Peptides, and Tuning of the Peptide Bond Geometry. C. A. Sanchez, C. Gadais, C.;Chaume, G.; Girard, S.; Chelain, E.; Brigaud, T. Org. Lett. 2021, 382-3874. Trifluoromethylated Proline Surrogates as Part of "Pro-Pro" Turn-Inducing Templates. Gadais, C.; Van holsbeeck, K.; Moors, S. L. C.; Buyst, D.; Feher, K.; Van Hecke, K.; Tourwe, D.; Brigaud, T.; Martin, C.; De Proft, F.; Pytkowicz, J.; Martins, J. C.; Chaume, G.; Ballet, S. ChemBioChem 2019, 20, 2513-2518.
5. CF2H as hydrogen bond donor group for the fine tuning of peptide bond geometry with difluoromethylated pseudoprolines. Malquin, N.; Rahgoshay, K.; Lensen, N.; Chaume, G.; Miclet, E.; Brigaud, T. Chem. Commun. 2019, 55, 12487-12490.
6. Trifluoromethylated proline analogues as efficient tools to enhance the hydrophobicity and to promote passive diffusion transport of the L-prolyl-L-leucyl glycinamide (PLG) tripeptide. Oliver, M.; Gadais, C.; Garcia-Pindado, J.; Teixido, M.; Lensen, N.; Chaume, G.; Brigaud, T. RSC Adv. 2018, 8, 14597-14602.
7. Probing the outstanding local hydrophobicity increase of peptide sequences induced by trifluoromethylated amino acids incorporation. Gadais, C.; Devillers, E.; Gasparik, V.; Chelain, E.; Pytkowicz, J.; Brigaud, T. ChemBioChem 2018, 19, 1026–1030.
8. Tailored Approaches towards the Synthesis of L-S-(Trifluoromethyl)cysteine- and L-Trifluoromethionine-Containing Peptides. Gadais, C.; Saraiva-Rosa, N.; Chelain, E.; Pytkowicz, J.; Brigaud, T. Eur. J. Org. Chem. 2017, 246-251.
9. Homochiral versus Heterochiral Trifluoromethylated Pseudoproline Containing Dipeptides: A Powerful Tool to Switch the Prolyl-Amide Bond Conformation. Chaume, G.; Simon, J.; Lensen, N.; Pytkowicz, J.; Brigaud, T.; Miclet, E. J. Org. Chem. 2017, 82, 13602-13608.10. Synthesis of protected enantiopure (R) and (S)-α-trifluoromethylalanine containing dipeptide building blocks ready to use for solid phase peptide synthesis. Devillers, E.; Pytkowicz, J.; Chelain, E.; Brigaud, T. Amino Acids 2016, 48, 1457-1468.
11. Incorporation of CF3−Pseudoprolines into Peptides: A Methodological Study, Chaume, G.; Simon, J.; Caupène, C.; Lensen, N.; Miclet, E.; Brigaud, T. J. Org. Chem. 2013, 78, 10144-10153.


