You are here :
- Unité de recherche
- BioCIS
- Home
- Research topics
- Fluorinated chiral compounds
- Fluorinated chiral auxiliaries
Fluorinated chiral auxiliaries
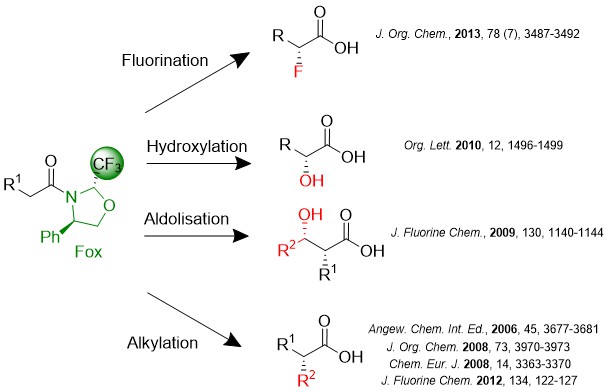
Our team develops the use of fluorinated chiral auxiliaries derived from trifluoromethylated oxazolidines (FOX) for the reactions of alkylation, hydroxylation or fluorination of amide enolates. The excellent diastereoselectivities obtained during these reactions as well as the possibility of using sterically hindered or functionalized substrates make this method a particularly effective way of accessing enantiopure acids, aldehydes or α-chiral alcohols. In addition, during the chiral auxiliary cleavage step, the latter is recovered with excellent yield. A theoretical and experimental study allowed us to highlight the presence of a fluor•••Metal interaction which stiffens the transition state of the reaction and orients the approach of the electrophile. This interaction competes with a π•••Metal interaction which leads to the formation of the same diastereomer thanks to the C2 pseudo-symmetry of the chiral auxiliary.
- Selected publications
-
1. Chiral 2-trifluoromethyl-4-phenyloxazolidine : A novel highly performing chiral auxiliary for amides alkylation, Tessier, A. ; Pytkowicz, J. ; Brigaud, T. Angew. Chem. Int. Ed. Engl. 2006, 45, 3677-3681.
2. Highly diastereoselective synthetic route to enantiopure β2-amino acids and γ-amino alcohols using a fluorinated oxazolidine (Fox) as chiral auxiliary, Tessier, A. ; Lahmar, N. ; Pytkowicz, J. ; Brigaud, T. J. Org. Chem. 2008, 73, 3970-3973.
3. Fluorine••• and π•••Alcali Metal Interactions control in the Stereoselective Amide Enolates Alkylation using Fluorinated Oxazolidines (Fox) as Chiral Auxiliary : An Experimental and Theoretical Study, Sini, G. ; Tessier, A. ; Pytkowicz, J. ; Brigaud, T. Chem. Eur. J. 2008, 14, 3363-3370.
4. Asymmetric aldol reactions using chiral CF3-Oxazolidines (Fox) as chiral auxiliary,Tessier, A. ; Pytkowicz, J. ; Brigaud, T. J. Fluorine Chem., 2009, 130, 1140-1144.
5. Highly Diastereoselective alfa-Hydroxylation of Fox Chiral Auxiliary-Based Amide Enolates with Molecular Oxygen, Lubin, H. ; Tessier, A. ; Chaume, G. ; Pytkowicz, J. ; Brigaud, T. Org. Lett. 2010, 12, 1496-1499.
6. 2-Trifluoromethyl-2-methyl-4-phenyloxazolidine: A new chiral auxiliary for highly diastereoselective enolate alkylation, Tessier, A.; Pytkowicz, J.; Brigaud, T. J Fluorine Chem. 2012, 134, 122-127
7. Crystallization-Induced Dynamic Resolution of Fox Chiral Auxiliary and Application to the Diastereoselective Electrophilic Fluorination of Amide Enolates. Lubin, H.; Dupuis, C.; Pytkowicz, J.; Brigaud, T. J. Org. Chem., 2013, 78 (7), 3487-3492


