You are here :
- Unité de recherche
- BioCIS
- Home
- Research topics
- Fluorinated chiral compounds
- Enantiopure fluorinated amino acids
Enantiopure fluorinated amino acids
Fluorinated amino acids are very interesting targets since they confer new physical and biological properties to the molecules in which they are incorporated. However, their preparation in enantiopure form remains a challenge, which explains their low use to date. Our laboratory has developed several access routes for the preparation of amino acids in enantiopure form, all based on the use of a common intermediate: chiral fluoromethylated oxazolidines (FOX). These particularly stable compounds are obtained from fluorinated derivatives and various commercial chiral amino alcohols (in general, (R)-phenylglycinol).
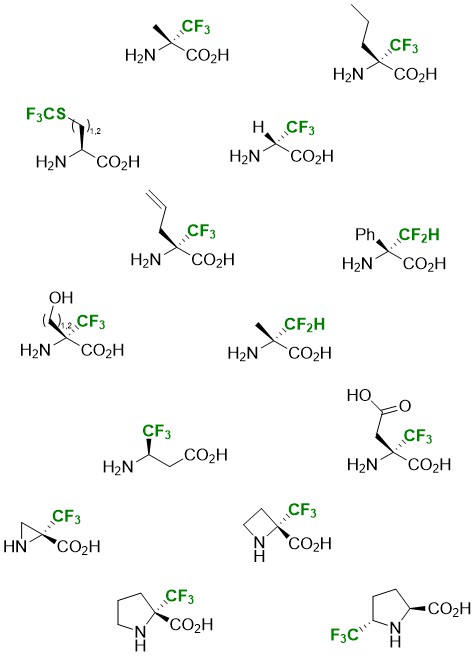
Strecker route
The first synthetic route is based on the implementation of a Strecker reaction. A methodological study was carried out using chiral trifluoromethylated imines or oxazolidines, by varying the nature of the Lewis acid and the chiral copula. Although the diastereoselectivity of the reaction is modest, the separation of the two diastereoisomers is simple. This strategy allowed the synthesis of (R)-(+)-trifluoroalanine as well as that of the two enantiomers of α-Tfm-alanine, α-Tfm-aspartic acid and α-Tfm-serine.
- Selected publications
-
1. Lewis acid activation of chiral 2-trifluoromethyl-1,3-oxazolidines. Application to the stereoselective synthesis of trifluoromethylated amines, alfa- and beta-amino acids, Lebouvier, N. ; Laroche, C. ; Huguenot, F. ; Brigaud, T. Tetrahedron Lett. 2002, 43, 2827-2830.
2. Concise synthesis of enantiopure alfa-trifluoromethyl alanines, diamines and amino alcohols via the Strecker-type reaction, Huguenot, F. ; Brigaud, T. J. Org. Chem. 2006, 71, 7075-7078.
3. Concise Synthesis of Enantiopure (S)- and (R)-alpha-Trifluoromethyl Aspartic Acid and alpha-Trifluoromethyl Serine from Chiral Trifluoromethyl Oxazolidines (Fox) via the Strecker-type Reaction, Simon, J.; Nguyen, T. T.; Chelain, E.; Lensen, N.; Pytkowicz, J.; Chaume, G.; Brigaud, T. Tetrahedron : Asymmetry 2011, 22, 309-314.
4. Straightforward Synthesis of Novel Enantiopure α-Trifluoromethylated Azetidine 2-Carboxylic Acid and Homoserines. Lensen, N.; Marais, J.; Brigaud, T. Org. Lett. 2015, 17, 342-345
5. Synthesis of Enantiopure trans-2,5-Disubstituted Trifluoromethylpyrrolidines and (2S,5R)‑5-Trifluoromethylproline. Lubin, H.; Pytkowicz, J.; Chaume, G.; Sizun-Thomé, G.; Brigaud, T. J. Org. Chem. 2015, 80, 2700-2708
6. Enantiopure α-Trifluoromethylated Aziridine-2-carboxylic Acid (α-TfmAzy): Synthesis and Peptide Coupling. Ouerfelli, O.; Simon, J.; Chelain, E.; Pytkowicz, J.; Besbes, R.; Brigaud, T. Org. Lett. 2020, 22, 2946-2949.
7. Synthesis of enantiopure α-Tfm-proline and α-Tfm-pipecolic acid from oxazolo-pyrrolidines and -piperidines. C. A. Sanchez, C. Gadais, S. Diarra, A. Bordessa, N. Lensen, E. Chelain and T. Brigaud, Org. Biomol. Chem., 2021, 6771-6775
Mannich/Reformatsky route
A Mannich-type reaction via the addition of a silylated ketene acetal in the presence of Lewis acid gives access, after separation of the diastereoisomers, to the corresponding (R)-β-trifluoromethyl-β-amino acid in enantiopure form. The use of Reformatsky's reagent provides α-Tfm-β-lactam, precursor of β-trifluoromethyl-β-amino acid.
- Selected publications
-
Convenient asymmetric synthesis of beta-trifluoromethyl-beta-amino acid, beta-amino ketones and gama-amino alcohols via Reformatsky and Mannich type reactions from 2-trifluoromethyl-1,3-oxazolidines, Huguenot, F. ; Brigaud, T. J. Org. Chem. 2006, 71, 2159-2162.
Allylsilane Route
We have developed a second route for the synthesis of original α-Tfm AAs based on the use of an α-trifluoromethylated allylmorpholinone. Several grams of this key intermediate can be obtained in two steps from the imine or oxazolidine derived from ethyl trifluoropyruvate via an allylation reaction. After separation of the two diastereoisomers, (S)-α-Tfm-α-allylglycine and (S)-α-Tfm-norvaline were obtained enantiopurely. The incorporation of the allylic chain also allowed the synthesis of various pyrrolidine motif α-Tfm AAs. A hydroboration-aminocyclization sequence allowed the synthesis of each enantiomer of α-Tfm-proline. The implementation of a hydroboration-oxidation-aminocyclization sequence leads to each enantiomer of α-Tfm-pyroglutamic. We have also described an iodocyclization reaction from allylmorpholinone which also leads to the two enantiomers of α-Tfm-proline. Intermediate iodine derivatives can be engaged in an elimination-dihydroxylation sequence to lead in a completely diastereoselective manner to enantiopure dihydroxylated α-Tfm-prolines.
- Selected publications
-
1. Straightforward synthesis of (S)- and (R)-alfa-trifluoromethyl proline from chiral oxazolidines derived from ethyl trifluoropyruvate, Chaume, G., Van Severen, M.-C., Marinkovic, S. ; Brigaud, T. Org. Lett. 2006, 8, 6123-6126.
2. Concise access to enantiopure (S)- and (R)-α-trifluoromethyl pyroglutamic acids from ethyl trifluoropyruvate-based chiral CF3-oxazolidines (Fox), Chaume, G. ; Van Severen, M. -C. ; Ricard, L. ; Brigaud, T. J. Fluorine Chem. 2008, 129, 1104-1109.
3. Iodocyclization of Chiral CF3-Allylmorpholinones : A Versatile Strategy for the Synthesis of Enantiopure alfa-Tfm-Prolines and alfa-Tfm-Dihydroxyprolines, Caupène, C. ; Chaume, G. ; Ricard, L. ; Brigaud, T. Org. Lett. 2009, 11, 209-212.
Difluoroénoxysilane route
In collaboration with the team of Professor Charles Portella (University of Reims Champagne Ardenne, UMR 6519), the reactivity of chiral difluorinated oxazolidines was studied to access α-Dfm-amino acids via the Strecker reaction. After separation of the diastereoisomers, (S)-α-Dfm-alanine and (R)-α-Dfm-alanine are obtained in enantiopure form.
- Selected publications
-
Umpolung reactivity of difluoroenol silyl ethers with amines and amino alcohols. Application to the synthesis of enantiopure alfa-difluoromethyl amines and amino acids, Huguenot, F. ; Billac, A. ; Brigaud, T. ; Portella, C. J. Org. Chem. 2008, 73, 2564-2569.


