You are here :
- Unité de recherche
- BioCIS
- Home
- Research topics
- Fluorinated chiral compounds
Synthesis of trifluoromethylated compounds
Our methodology relies on the use of a common intermediate: FOX chiral fluoromethylated oxazolidines. The implementation of a Strecker type reaction makes it possible to introduce the nitrile group and offers a direct access route to the corresponding α-Tfm-amino acids, α-Tfm-amino alcohols or α-Tfm-diamines. A Mannich-type addition gives access to β-Tfm-β-amino acid, β-Tfm-β-aminoketone or γ-Tfm-γ-aminoalcohol derivatives. Reformatsky's reagent provides α-Tfm-β-lactam, precursor of β-Tfm-β-amino acids. Finally, the oxazolidine allylation or alkynylation reactions lead to homoallylic α-Tfm-amine and propargyl α-Tfm-amine intermediates.
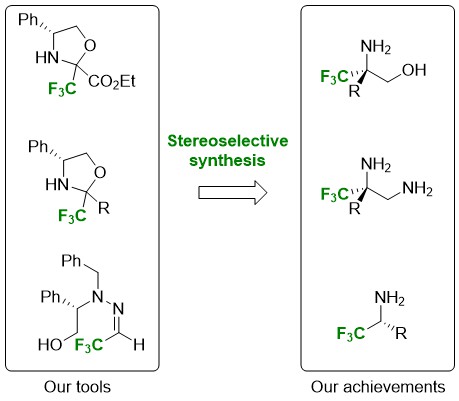
The stereoselectivities observed for all of these reactions vary but, in the vast majority of cases, the conformations induced by the trifluoromethyl group allow us to easily separate the diastereoisomers and to obtain enantiomerically pure α-trifluoromethylated compounds. Our laboratory has also developed an original method for the preparation of α-Tfm-amines. The additions of organometallic reagents of the Grignards or lithium type to a hydrazone derived from fluoral have proven to be highly stereoselective. This stereoselectivity can be explained by the formation of a six-center intermediate involving chelation of free hydroxyl and nitrogen.
- Selected publications
-
1. Highly Diastereoselective Synthesis of Enantiopure β-Trifluoromethyl β-Amino Alcohols from Chiral Trifluoromethyl Oxazolidines (Fox), Simon, J.; Chelain, E.; Brigaud, T. Org. Lett., 2012, 14 (2), 604–607.
2. Straightforward synthesis of enantiopure (R)- and (S)-trifluoroalaninol, Pytkowicz, J. ; Stephany, O. ; Marinkovic, S. ; Inagaki, S. ; Brigaud, T. Org. Biomol. Chem. 2010, 8, 4540-4542.
3. Concise synthesis of enantiopure α-trifluoromethyl alanines, diamines and amino alcohols via the Strecker-type reaction, Huguenot, F. ; Brigaud, T. J. Org. Chem. 2006, 71, 7075-7078.
4. Highly diastereoselective addition of organometallic reagents to a trifluoroacetaldehyde hydrazone derived from (R)-N-benzylphenylglycinol, Fries, S.; Pytkowicz, J.; Brigaud, T. Tetrahedron Lett. 2005, 45, 4761-4764.
5. Lewis acid activation of chiral 2-trifluoromethyl-1,3-oxazolidines. Application to the stereoselective synthesis of trifluoromethylated amines, α- and β-amino acids, Lebouvier, N. ; Laroche, C. ; Huguenot, F. ; Brigaud, T. Tetrahedron Lett. 2002, 43, 2827-2830
In collaboration with the team of Professor Charles Portella (University of Reims Champagne Ardenne, UMR 6519), the nucleophilic and electrophilic reactivities of difluoroenoxysilanes have also been studied in order to access various chiral difluorinated compounds.
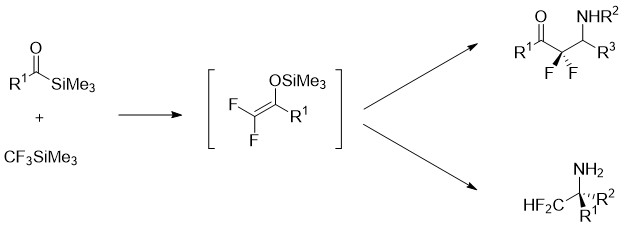
Difluoroenoxysilanes obtained from acylsilanes and CF3SiMe3 are nucleophilic reagents. Their reaction with chiral imines and oxazolidines leads to chiral difluoro-β-aminoketones. Difluoroenoxysilanes also exhibit electrophilic reactivity. Their reaction with chiral amino alcohols results in chiral difluorinated oxazolidines. These oxazolidines are building blocks of choice for accessing difluoroamines and difluoroamino acids in enantiopure form.
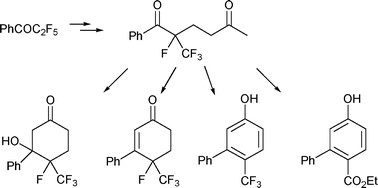
The action of a tetrafluoroenoxysilane on the methylvinylketone leads by Robinson annelation reaction to obtaining derivatives of trifluoromethyl-cyclohexanone, trifluoromethyl-cyclohexenone and trifluoromethyl-phenol.
- Selected publications
-
1. Synthesis of trifluoromethyl cyclohexyl, cyclohexenyl and aryl compounds via stepwise Robinson annulation, Massicot, F.; Iriarte, A. M.; Brigaud, T.; Lebrun, A.; Portella, C. Org. Biomol. Chem. 2011, 2, 600-603.
2. Umpolung Reactivity of Difluoroenol Silyl Ethers with Amines and Amino Alcohols. Application to the Synthesis of Enantiopure α-Difluoromethyl Amines and Amino Acids, Huguenot, F. ; Billac, A. ; Brigaud, T. ; Portella, C. J. Org. Chem. 2008, 73, 2564-2569.
3. Mild Synthesis of β-Amino-α,α-difluoro Ketones from Acylsilanes and Trifluoromethyltrimethylsilane in a One-Pot Imino Aldol Reaction, Jonet, S. ; Cherouvrier, F. ; Brigaud, T. ; Portella, C. Eur. J. Org. Chem. 2005, 20, 4304-4312.


